
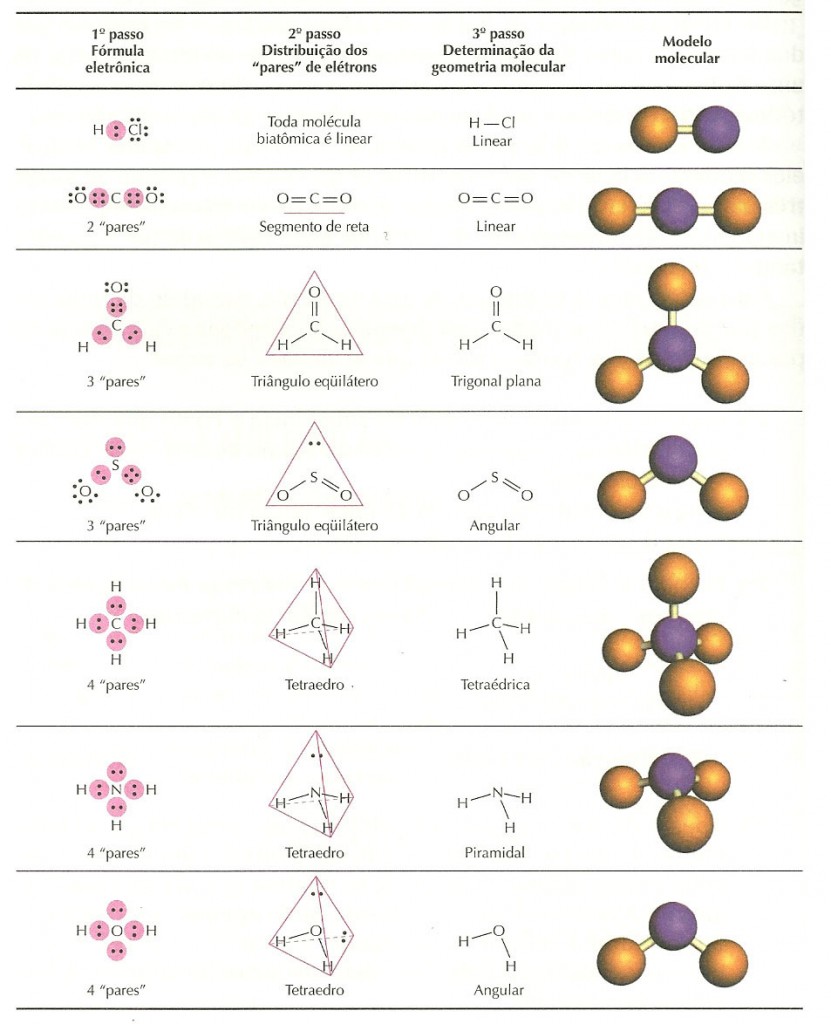
Molecular structure describes the location of the atoms, not the electrons. The electron-pair geometries shown in describe all regions where electrons are located, bonds as well as lone pairs. It is important to note that electron-pair geometry around a central atom is not the same thing as its molecular structure. To determine the molecular geometry of a molecule, one must first determine the electronic geometry by drawing the Lewis structure. Electron-pair Geometry versus Molecular Structure. due to the Lewis structure of C F4, The general molecular geometry formula for C F4 is AX4. We know that carbon is the core atom, with four electron pairs bound (four C- F) and zero lone pairs. This is in contrast to the electronic geometry, which describes the shape of all electron regions. The lone pairs of electrons on the center carbon atom are denoted by the letter N.
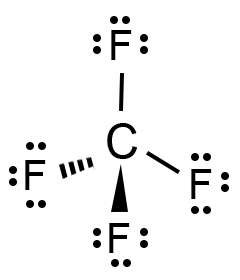

The band theory is a form of molecular orbital theory. geometries: Electronic Geometry Molecular Geometry linear linear trigonal. Show transcribed image text Which of the following has a molecular structure best described as involving an "incomplete octet"? CF4 F2 BF3 OF2 NF3 What is the correct molecular geometry for SeBr3 ? trigonal pyramidal tetrahedral bent -shaped trigonal planar Which of the following statements is INCORRECT? Delocalized pi orbitals are formed when electrons are shared by unhybridized p orbitals more than two atoms. (a) The molecule, tetrafluoromethane (CF4), is nonpolar because its four polar. The valence band is at higher energy than the conduction band.
fluorine it is -1 CF4 molecule is neutral, therefore, the oxidation state of carbon is + 4. CF4, the outside of the molecule is uniformly negative, while the inside. b) choosing the central atom of the molecular structure. The band theory is a form of molecular orbital theory. Chapter 5: Electron Configuration, Lewis Dot Structure, and Molecular Shape. CH2Cl2 Molecular geometry Valence electrons Electron geometry Lewis Structure Molecular geometry 3. CF4 Lewis Structure Molecular geometry Valence electrons Electron geometry Lewis Structure 2. Transcribed image text: Electron geometry 1. Which of the following has a molecular structure best described as involving an "incomplete octet"? CF4 F2 BF3 OF2 NF3 What is the correct molecular geometry for SeBr3 ? trigonal pyramidal tetrahedral bent -shaped trigonal planar Which of the following statements is INCORRECT? Delocalized pi orbitals are formed when electrons are shared by unhybridized p orbitals more than two atoms. Answer: part - (1): Lewis structure of CF4, is in Explanation, below.


 0 kommentar(er)
0 kommentar(er)
